-
 play_arrow
play_arrow
BayRadio Listen Live Broadcasting in Spain
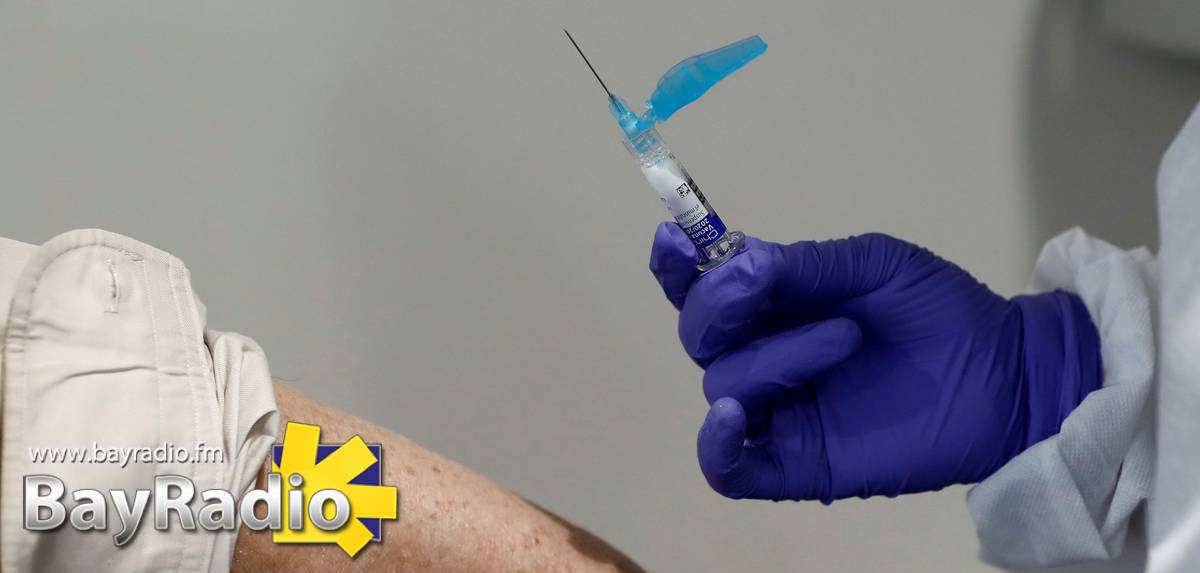
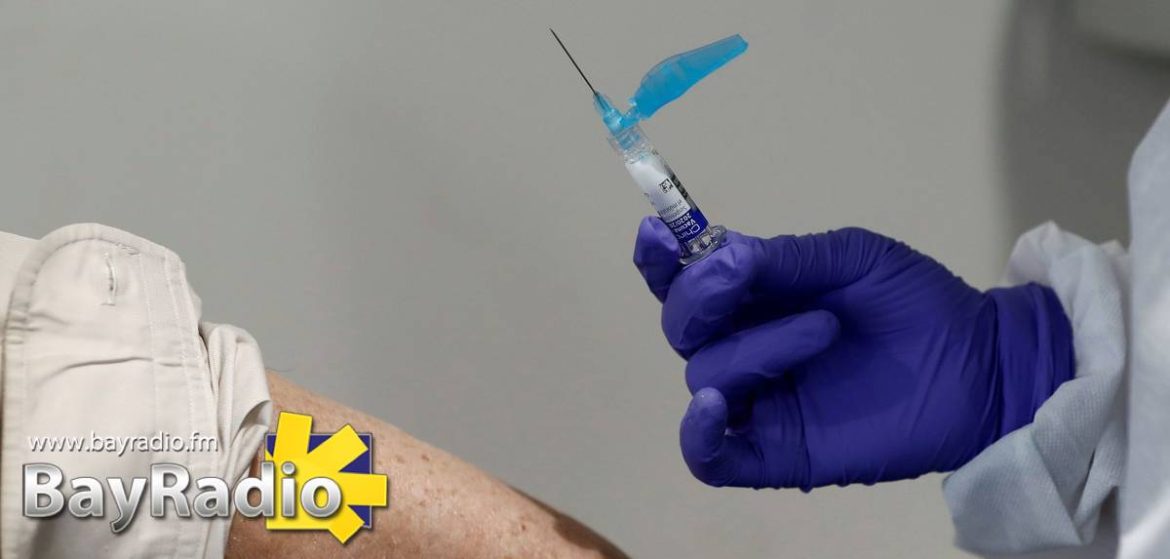
Spain’s Ministerio de Salud is looking for thousands of volunteers to take part in the country’s first late-stage vaccine trials.
Spain’s drug approval agency (AEMPS) has authorised a Phase 3 clinical trial of the COVID-19 vaccine developed by Janssen, a subsidiary of Johnson & Johnson.
This is the first late-stage trial to go ahead in Spain.
The ministry said two doses of the real vaccine and placebos will be administered to 30,000 volunteers recruited in nine countries: Spain, Belgium, Colombia, France, Germany, the Philippines, South Africa, UK and the US.
A total of 3,000 volunteers will be sought in Spain, according to statements yesterday from Spain’s minister of health, Salvador Illa.
"La @AEMPSGOB ha autorizado el ensayo clínico fase III en España de la vacuna contra la #COVID19 de Janssen. Se trata del primer ensayo fase III para una #vacuna de COVID-19 autorizado de este tipo en España. Se hará en 8 hospitales con 3.000 voluntarios" pic.twitter.com/EnxzEwLA8a
— Ministerio de Sanidad (@sanidadgob) November 18, 2020
Eight Spanish hospitals – in Madrid, Barcelona and Pamplona – will participate and start searching for volunteers ‘as soon as possible’.
The Janssen vaccine is based on a common cold virus, which has been modified with genetic information from the coronavirus in a bid to get the body to create specific defences without posing any health risk.
Only volunteers without pre-existing medical conditions associated with a risk of developing serious COVID-19 symptoms will be admitted in the initial stage of testing.
Written by: BayRadio News
Similar posts
Recent Posts
- Robotic Surgery for Prostate Cancer: What Is Radical Prostatectomy and How Does the Da Vinci Robot Improve It
- What Is Fibromyalgia? Symptoms and Treatments of an Invisible Illness That Requires Specialized Attention
- AMASVISTA Glass: 10 reasons to choose SUNFLEX glass curtains
- Robotic Surgery, Immunotherapy and Comprehensive Care Take Centre Stage at Pancreatic Cancer Conference at Quirónsalud Torrevieja
- Robotic Surgery Against Ovarian Cancer: Greater Precision, Less Pain and Faster Recovery

Ctra. Cabo La Nao, CC La Nao, Local 6 03730 Javea, Alicante, Spain
Advertise with us
Do you have a business in Spain? Do you provide a service to the expat community in Spain? Would you like your message to reach over 500.000 people on a weekly basis?
BayRadio is a community orientated radio station offering fantastic content to our many listeners and followers across our various platforms. Contact us now and find out what Bay can do for you!
Our business is helping your business grow.
BAY RADIO S.L. © 2024. ALL RIGHTS RESERVED. WEB DESIGN BY MEDIANIC







